The goal of our work is to identify genetic and protein networks that orchestrate telomere and genomic stability that may be targetable in cancer therapy. To achieve this we combine cellular imaging and proteomic platforms that provide a basis for further molecular dissection of the mechanisms involved in telomere dysfunction in human disease. Current projects in the lab include;
1. IDENTIFICATION OF FACTORS INVOLVED IN THE INDUCTION OF THE ALT PATHWAY
The Alternative Lengthening of Telomeres, ALT pathway is a mechanism of telomere length maintenance that is active in ~10% of human cancers. Many of these cancers affect the Central Nervous System and Pancreatic Neuroendocrine systems and unfortunately patients tend to have a poor prognosis. Since it was first identified in human cancers significant strides have been made in understanding the molecular biology of ALT. However, how the ALT pathway is activated has remained one of the most enigmatic aspects of telomere biology.
Evidence had suggested that ALT induction may be the result of an atypical chromatin structure at telomeres. As a postdoc, Roddy discovered a system with which to rapidly induce ALT. This involves depletion of the histone chaperones, ASF1a and ASF1b which coordinate the shuttling of histone proteins during DNA replication. This experimental system allows, for the first time, the direct identification of factors required for ALT activation.
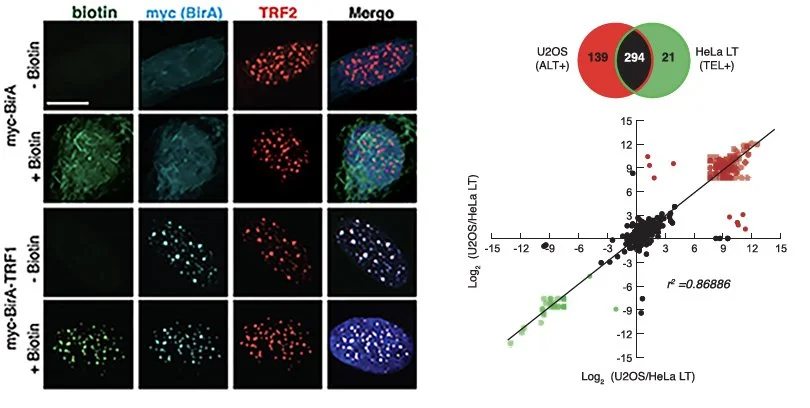
As an independent lab, our initial goal was to identify new factors involved in ALT. To do this, we decided to implement proteomics based protein discovery. This approach known as BioID involved delivering protein labelling enzymes to telomeres. These enzymes (BirA) puts a tag on proteins that it is close to. In this case, by putting BirA at telomeres we could mark all the proteins at telomeres. This worked out very well and allowed us to identify and characterize several important mediators of ALT - PolH, RAD51AP1, PARP, MSH2-6 and several more.
Source. Garcia-Exposito et al., Cell Reports (2016)