Telomeres are crucial structures that are located at the tip of every chromosome in a cell. They are distinct in so far as they are composed of the following DNA sequence, TTAGGG, which is repeated several hundred times. Though they mostly contain double stranded TTAGGG repeats the very end has a protruding single strand of TTAGGG repeats called an "Overhang". This overhang is manipulated by a protein complex called Shelterin into a lasso-type structure called a T-Loop. The T-Loop provides the cell with a mechanism to hide the ends of each chromosome.
Above: Left to Right;
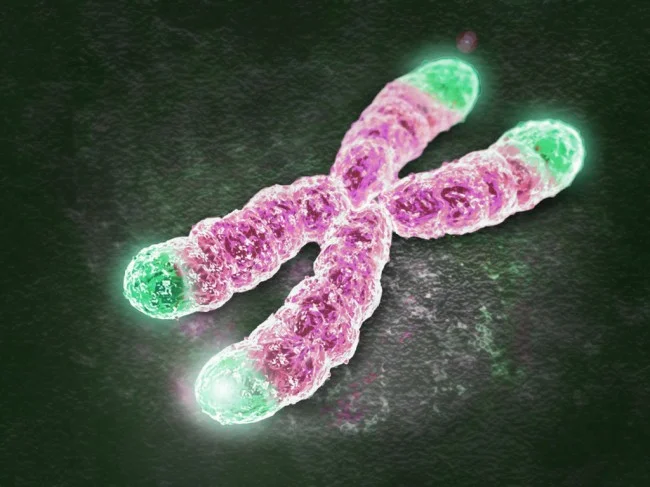
- Artistic representation of telomeres (green) on a metaphase chromosome (purple).
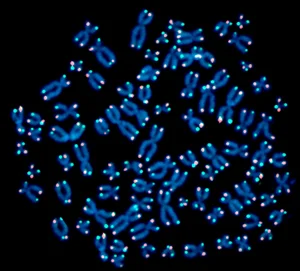
- The real thing. Telomeres (red & green dots) at the ends of human chromosomes (blue)
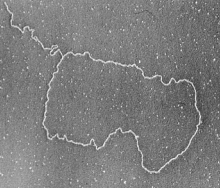
- Visualization of T-Loops by Electron Microscopy. (Image by Jack Griffith & Titia de Lange, Published in Cell, 1999)
Telomeres serve 3 primary functions that are important for healthy cells.
1. By tucking away the exposed terminal end of TTAGGG repeat sequence into a T-Loop, telomeres prevent unwarranted activity of DNA repair mechanisms. Should DNA repair mechanisms get access to telomeres, one possible outcome is the fusion of telomeres that are located on different chromosomes. This is a catastrophic event and can lead to cancer.
2. Every time the cell replicates a small fragment of the telomeric DNA is lost. This is called "The End-Replication" problem. Over the course of many generations of cell division telomeres are shortened to a threshold called "The Hayflick Limit". At the Hayflick Limit the cell ceases to divide and enters a permanent state of arrest known as "Replicative Senescence". This process has been adapted by the cell as an effective barrier to the excessive cell growth that is often associated with cancer. Therefore, telomere shortening can actually help to prevent cancer.
3. Some critical groups of cells, like stem and germ cells, do not undergo telomere shortening and replicative senescence. This is because they have activated an enzyme known as Telomerase. This remarkable enzyme maintains healthy telomeres by adding back TTTAGGG repeats to the end of chromosomes. Therefore, the hayflick limit is not encountered and the cell remains healthy and fully functional. In fact, if activated or experimentally introduced into a cell that has been aged in the lab or taken from old individuals, and thus has short telomeres, telomerase can heal those telomeres and rejuvenate the cell. Consequently, telomerase has been the focus of intensive efforts to counteract the onset of age-associated diseases and enable healthy aging.
In 2009, for their research that led to the discovery of Telomerase, Elizabeth Blackburn, Carol Greider and Jack Szostak were awarded the Nobel Prize in Physiology or Medicine. Hit the link below for more details.
Our lab is interested in the factors that maintain the telomeric chromatin environment and its role in ALT cancer induction.